WEB & MOBILE
Lorem ipsum dolor sit amet, dolor adipisicing dolor sit amet elit atis unde o
Reso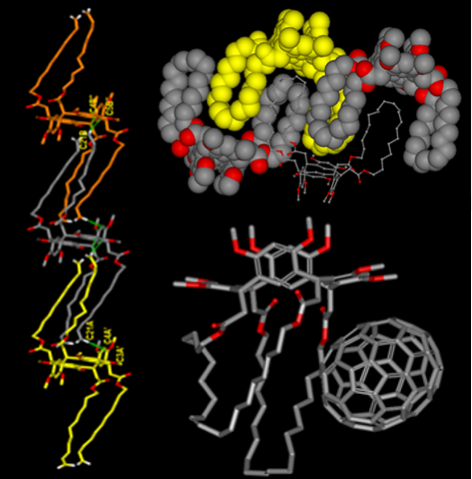 rc[4]arenes are macrocyclic molecules containing resorcinol rings bridged by methylene groups and are among the most ubiquitous host molecules in supramolecular chemistry. They are obtained from the acid-catalyzed condensation reaction between aliphatic aldehyde and resorcinol. The macrocyclic ring can adopt five extreme symmetrical arrangements: cone, flattened cone, diamond or 1,2-alternate, saddle or 1,3-alternate, and chair conformation. The most common conformation of resorc[4]arenes (cone) is very close in solution to that one of calixarenes, but it is, in reality, the result of the equilibrium between two equivalent forms. These compounds are characterized by an hydrophobic cavity which allows the interactions with neutral molecules but they can also be derivatized in the lower rim or in the upper rim with other functional groups which favor interactions with ions or other molecules. Inter alia we designed, synthesized and studied C-alkyl resorc[4]arenes that are able to form a complex with cations such as Cu(II), Fe(III) or Ga(III) to recognize chiral amino acids, to entrap nitrosonium (NO+) cations, and to interact with protein surfaces. We previously designed, resorc[4]arene octamethyl ethers able to entrap nitrosonium (NO+) cation in their cavities. As an extension of our studies on NO+ entrapment, with the aim of combining the entrapping properties of resorcarenes with the possible anchorage to a solid support, we planned to synthesize resorc[4]arenes featuring long aliphatic side chains ending with a vinylidene group. However, looking at the terminal double bounds of the four alkyl chains, we had the serendipitous idea to submit the above-mentioned resorca[4]arenes to a metathesis reaction, in order to incorporate the macrocycles into polymeric architectures with intriguing mechanical properties. Resorc[4]arenes, are widely used in supramolecular chemistry, thanks to the presence of lower and upper rims, which can be modified in order to form Host-Guest interactions. Specific guest molecules, such as drugs and proteins can be bind to Resorc[4]arene-host-cavity, making interesting complexes applied in the development of drug-delivery and biosensor systems.
rc[4]arenes are macrocyclic molecules containing resorcinol rings bridged by methylene groups and are among the most ubiquitous host molecules in supramolecular chemistry. They are obtained from the acid-catalyzed condensation reaction between aliphatic aldehyde and resorcinol. The macrocyclic ring can adopt five extreme symmetrical arrangements: cone, flattened cone, diamond or 1,2-alternate, saddle or 1,3-alternate, and chair conformation. The most common conformation of resorc[4]arenes (cone) is very close in solution to that one of calixarenes, but it is, in reality, the result of the equilibrium between two equivalent forms. These compounds are characterized by an hydrophobic cavity which allows the interactions with neutral molecules but they can also be derivatized in the lower rim or in the upper rim with other functional groups which favor interactions with ions or other molecules. Inter alia we designed, synthesized and studied C-alkyl resorc[4]arenes that are able to form a complex with cations such as Cu(II), Fe(III) or Ga(III) to recognize chiral amino acids, to entrap nitrosonium (NO+) cations, and to interact with protein surfaces. We previously designed, resorc[4]arene octamethyl ethers able to entrap nitrosonium (NO+) cation in their cavities. As an extension of our studies on NO+ entrapment, with the aim of combining the entrapping properties of resorcarenes with the possible anchorage to a solid support, we planned to synthesize resorc[4]arenes featuring long aliphatic side chains ending with a vinylidene group. However, looking at the terminal double bounds of the four alkyl chains, we had the serendipitous idea to submit the above-mentioned resorca[4]arenes to a metathesis reaction, in order to incorporate the macrocycles into polymeric architectures with intriguing mechanical properties. Resorc[4]arenes, are widely used in supramolecular chemistry, thanks to the presence of lower and upper rims, which can be modified in order to form Host-Guest interactions. Specific guest molecules, such as drugs and proteins can be bind to Resorc[4]arene-host-cavity, making interesting complexes applied in the development of drug-delivery and biosensor systems.
As an extension of Host-Guest studies, with the aim of combining the entrapping properties of resorcarenes with the possible synthesis of 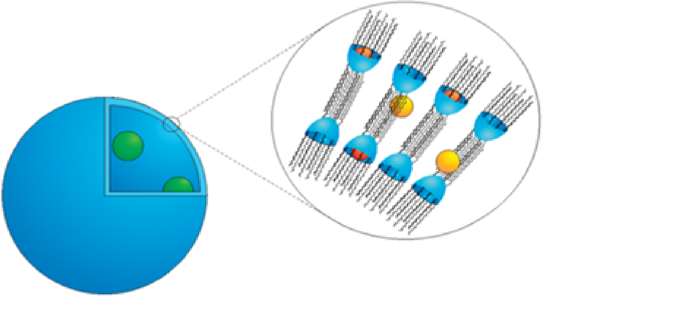 vesicles assembled in water as carriers for drug delivery applications, neutral cavitands based on Polyethylene glycol (PEG) groups attached at the upper rim will be synthesized. These kind of vesicles, assembled from deep cavitands in water act as hosts for three different types of guests: the cavitands bind small guest molecules, the bilayer attracts larger hydrophobic guests and the inner aqueous compartment contains hydrophilic molecules. The ability of these vesicles to behave as carriers of the natural product GlaB, capable of targeting the Hedgehog (Hh) pathway, and other molecules of therapeutic relevance will be checked.
vesicles assembled in water as carriers for drug delivery applications, neutral cavitands based on Polyethylene glycol (PEG) groups attached at the upper rim will be synthesized. These kind of vesicles, assembled from deep cavitands in water act as hosts for three different types of guests: the cavitands bind small guest molecules, the bilayer attracts larger hydrophobic guests and the inner aqueous compartment contains hydrophilic molecules. The ability of these vesicles to behave as carriers of the natural product GlaB, capable of targeting the Hedgehog (Hh) pathway, and other molecules of therapeutic relevance will be checked.
Copyright © Rebek et al, Chem. Commun., 2012, 48, 9251-9253
Biosensors are analytical devices in which the biochemical reaction is coupled to a transducer. One of the main goal in the development of 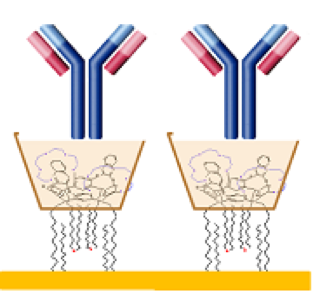 biosensors is to cover its surface with a high density and good orientation of proteins for the improvement of the performance enhancement. The design of Resorc[4]arene derivatives have been proposed as an alternative tool to immobilize proteins as biological component of ligand-based biosensor. Accordingly, the aim of this project is the synthesis and the characterization of new Resorc[4]arene-based systems as an artificial linker for enzymes and antibodies immobilization. The immobilization characteristics and surface coverage of adsorbed protein have been evaluated by Surface Plasmon Resonance (SPR).
biosensors is to cover its surface with a high density and good orientation of proteins for the improvement of the performance enhancement. The design of Resorc[4]arene derivatives have been proposed as an alternative tool to immobilize proteins as biological component of ligand-based biosensor. Accordingly, the aim of this project is the synthesis and the characterization of new Resorc[4]arene-based systems as an artificial linker for enzymes and antibodies immobilization. The immobilization characteristics and surface coverage of adsorbed protein have been evaluated by Surface Plasmon Resonance (SPR).
Resorc[4]arenes able to behave as chiral Host have been synthesized and the intrinsic enantioselectivity of the new receptors toward Guest molecules has been tested.
Synthesis of Bromoundecyl Resorc[4]arenes and Applications of the Cone Stereoisomer as Selector for Liquid Chromatography
As an extension of our studies on the multifaceted properties of C-alkylated resorc[4]arenes, we planned to immobilize resorc[4]arenes 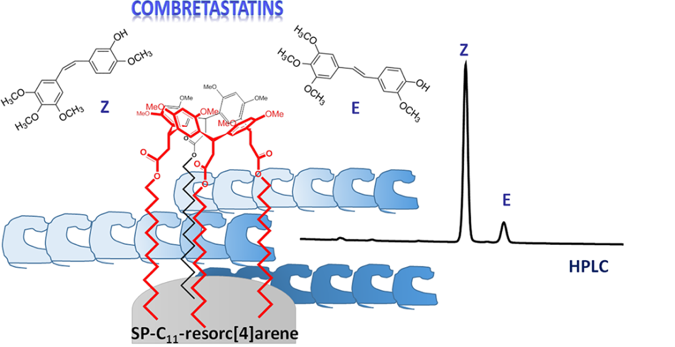 featuring C11 aliphatic side chains in the lower rim on a solid support. To this purpose, we synthesized two conformationally diverse resorc[4]arenes containing a bromoundecyl moiety in the four axial pendants. The cone stereoisomer 6a (30% yield) was selected for the reaction with an aminopropylated silica gel (APSG) obtained from spherical Kromasil Si 100, 5 μm particles, to give the corresponding immobilized SP-C11-resorc[4]arene system. The resulting polar-embedded stationary phase was fully characterized and investigated in the HPLC discrimination of the E/Z stereoisomers of naturally occurring and semi-synthetic combretastatins, a family of (Z)- stilbene anticancer drugs. The chair stereoisomer 6b (20% yield), when submitted to X-ray diffraction analysis, showed a peculiar self-assembly in the crystal lattice, forming intercalated polar and hydrophobic layers as a consequence of intermolecular Br・・・O halogen-bond interactions, according to a unique stacking motif. The potential and versatility of the SP-C11-resorc[4]arene stationary phase were shown as well in the separation of highly polar natural products, such as flavonoids, under reversed-phase (RP) conditions, and of fullerenes C60 and C70, by using apolar solvents as mobile phases.
featuring C11 aliphatic side chains in the lower rim on a solid support. To this purpose, we synthesized two conformationally diverse resorc[4]arenes containing a bromoundecyl moiety in the four axial pendants. The cone stereoisomer 6a (30% yield) was selected for the reaction with an aminopropylated silica gel (APSG) obtained from spherical Kromasil Si 100, 5 μm particles, to give the corresponding immobilized SP-C11-resorc[4]arene system. The resulting polar-embedded stationary phase was fully characterized and investigated in the HPLC discrimination of the E/Z stereoisomers of naturally occurring and semi-synthetic combretastatins, a family of (Z)- stilbene anticancer drugs. The chair stereoisomer 6b (20% yield), when submitted to X-ray diffraction analysis, showed a peculiar self-assembly in the crystal lattice, forming intercalated polar and hydrophobic layers as a consequence of intermolecular Br・・・O halogen-bond interactions, according to a unique stacking motif. The potential and versatility of the SP-C11-resorc[4]arene stationary phase were shown as well in the separation of highly polar natural products, such as flavonoids, under reversed-phase (RP) conditions, and of fullerenes C60 and C70, by using apolar solvents as mobile phases.
(J. Org. Chem., (2018), 83(15):7683-7693)
Nitrosonium Complexes of Resorc[4]arenes: Spectral, Kinetic, and Theoretical Studies
Reaction of Nitrosonium Cation with Resorc[4]arenes Activated by Supramolecular Control: Covalent Bond Formation: Resorc[4]arenes 1 and 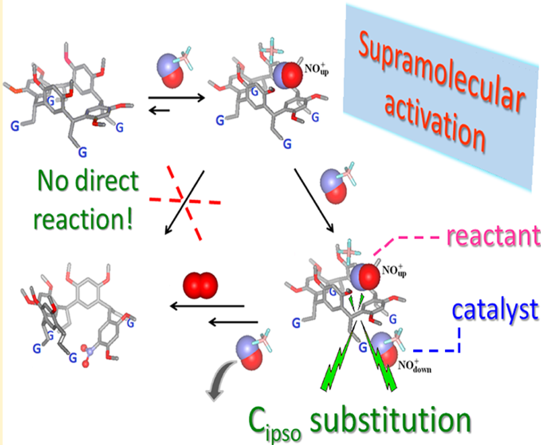 2, which previously proved to entrap NO+ cation within their cavities under conditions of host-to-guest excess, were treated with a 10-fold excess of NOBF4 salt in chloroform. Kinetic and spectral UV−visible analyses revealed the formation of isomeric 1:2 complexes as a direct evolution of the previously observed event. Accordingly, three-body 1−(NO+)2 and 2−(NO+)2 adducts were built by MM and fully optimized by DFT calculations at the B3LYP/6-31G(d) level of theory. Notably, covalent nitration products 4, 5 and 6, 7 were obtained by reaction of NOBF4 salt with host 1 and 2, respectively, involving macrocycle ring-opening and insertion of a nitro group in one of the four aromatic rings. In particular, compounds 4 and 6, both containing a trans-double bond in the place of the methine bridge, were oxidized to aldehydes 5 and 7, respectively, after addition of water to the reaction mixture. Calculation of the charge and frontier orbitals of the aromatic donor (HOMO) and the NO+ acceptor (LUMO) clearly suggests an ipso electrophilic attack by a first NO+ unit on the resorcinol ring, mediated by the second NO+ unit.
2, which previously proved to entrap NO+ cation within their cavities under conditions of host-to-guest excess, were treated with a 10-fold excess of NOBF4 salt in chloroform. Kinetic and spectral UV−visible analyses revealed the formation of isomeric 1:2 complexes as a direct evolution of the previously observed event. Accordingly, three-body 1−(NO+)2 and 2−(NO+)2 adducts were built by MM and fully optimized by DFT calculations at the B3LYP/6-31G(d) level of theory. Notably, covalent nitration products 4, 5 and 6, 7 were obtained by reaction of NOBF4 salt with host 1 and 2, respectively, involving macrocycle ring-opening and insertion of a nitro group in one of the four aromatic rings. In particular, compounds 4 and 6, both containing a trans-double bond in the place of the methine bridge, were oxidized to aldehydes 5 and 7, respectively, after addition of water to the reaction mixture. Calculation of the charge and frontier orbitals of the aromatic donor (HOMO) and the NO+ acceptor (LUMO) clearly suggests an ipso electrophilic attack by a first NO+ unit on the resorcinol ring, mediated by the second NO+ unit.
(J. Org. Chem. 2013, 78, 6935−6946)
Undecenyl resorc[4]arene was submitted to intramolecular and intermolecular olefin metathesis reaction in order to achieve the synthesis of a range of novel cage resorc[4]arenes, and the study of the docking with the fullerene family.
First Detection of a Ruthenium–Carbene–Resorc[4]arene Complex During the Progress of a Metathesis Reaction: For the first time we describe the detection of a ruthenium-carbene-resorc[4]arene complex produced, as a key intermediate, during an olefin metathesis reaction 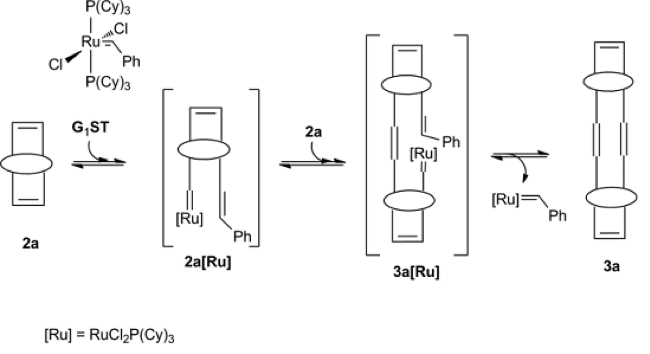 carried out on a resorc[4]arene bicyclic olefin with [Ru(=CHPh)Cl2(PCy3)2] Grubbs first-generation (G1ST) catalyst. The complex was identified by using high-resolution (600 MHz) 1H, 31P NMR and DOSY spectroscopy, in a non-invasive fashion. The singlet at δ = 19.98 ppm (1H NMR), attributed to the alkylidene proton in the G1ST catalyst and that at δ = 53.37 ppm (31P NMR), attributed to the phosphorus atom coordinated with the metal, were selected as probes for a kinetic analysis. The intensity of these signals decreased at the expense of the singlets at 19.26 (1H NMR) and 52.68 ppm (31P NMR), diagnostic for the formation of the ruthenium-carbene-resorc[4]arene complex 3a[Ru]. The resorc[4]arene activated olefin proved to behave as a key propagating species leading to oligomers according to a ROMP pathway.
carried out on a resorc[4]arene bicyclic olefin with [Ru(=CHPh)Cl2(PCy3)2] Grubbs first-generation (G1ST) catalyst. The complex was identified by using high-resolution (600 MHz) 1H, 31P NMR and DOSY spectroscopy, in a non-invasive fashion. The singlet at δ = 19.98 ppm (1H NMR), attributed to the alkylidene proton in the G1ST catalyst and that at δ = 53.37 ppm (31P NMR), attributed to the phosphorus atom coordinated with the metal, were selected as probes for a kinetic analysis. The intensity of these signals decreased at the expense of the singlets at 19.26 (1H NMR) and 52.68 ppm (31P NMR), diagnostic for the formation of the ruthenium-carbene-resorc[4]arene complex 3a[Ru]. The resorc[4]arene activated olefin proved to behave as a key propagating species leading to oligomers according to a ROMP pathway.
(Eur. J. Org. Chem. 2017, 2407–2415).
Synthesis of a Double-Spanned Resorc[4]arene via Ring-Closing Metathesis and Calculation of Aggregation Propensity : Ring-closing metathesis (RCM) catalyzed by a second-generation Grubbs catalyst has been used to synt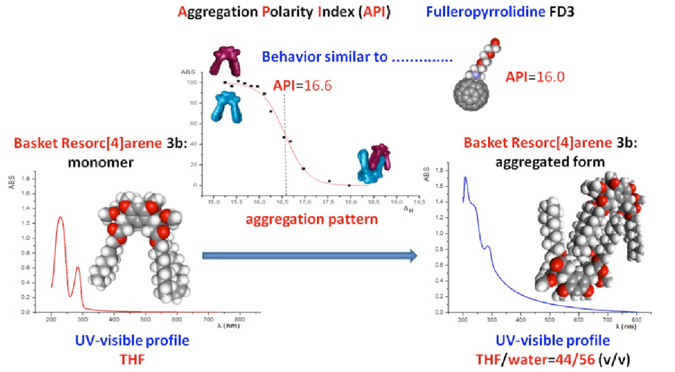 hesize resorc[4]arenes 2b−5b starting from undecenyl resorc[4]arene 1b fixed in the cone conformation. X-ray diffraction analysis of the major metathesis product, 3b (50% yield), revealed a cavity-shaped architecture resembling a basket, endowed with a large intramolecular space (∼10 Å) and a strong propensity to self-assemble as a supramolecular trio of heterochiral dimers. This prompted us to investigate the aggregation propensity of basket 3b in THF/water solution by UV−visible spectroscopy. The cavitation Gibbs free-energy change (ΔΔGcav = 4.78 kcal mol−1) associated with the self-assembly of macrocycle 3b was calculated as a measure of the solvophobic interactions involved in the process.
hesize resorc[4]arenes 2b−5b starting from undecenyl resorc[4]arene 1b fixed in the cone conformation. X-ray diffraction analysis of the major metathesis product, 3b (50% yield), revealed a cavity-shaped architecture resembling a basket, endowed with a large intramolecular space (∼10 Å) and a strong propensity to self-assemble as a supramolecular trio of heterochiral dimers. This prompted us to investigate the aggregation propensity of basket 3b in THF/water solution by UV−visible spectroscopy. The cavitation Gibbs free-energy change (ΔΔGcav = 4.78 kcal mol−1) associated with the self-assembly of macrocycle 3b was calculated as a measure of the solvophobic interactions involved in the process.
(J. Org. Chem., 2014, 79 (22), 11051–11060)
Undecenyl resorc[4]arene in the chair conformation as preorganized synthon for olefin metathesis: Tetramerization of (E)-2,4-dimethoxycinnamic acid v-undecenyl ester with ethereal BF3 gave three stereoisomers 1a, 1b, and 1c, which were assigned as the chair, cone, and 1,2-alternate conformations, respectively. The cha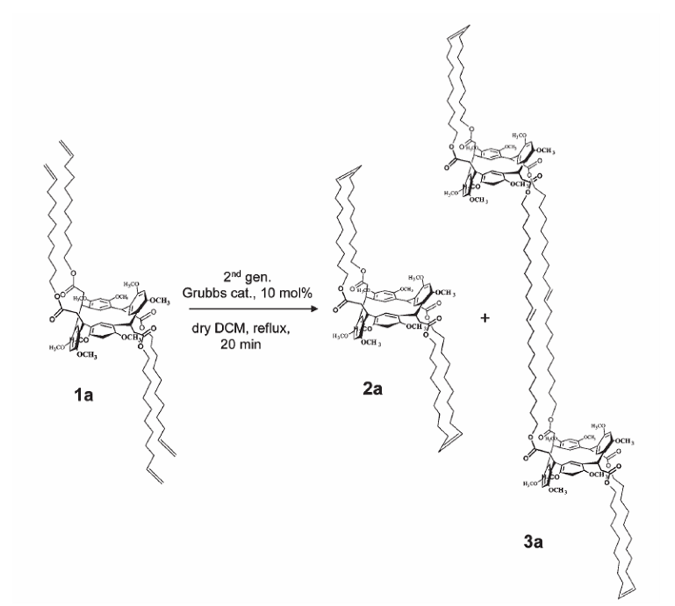 ir conformation of 1a was confirmed by X-ray diffraction analysis, which also showed a peculiar self-assembly behavior in the crystal lattice, forming intercalated hydrophilic and hydrophobic layers (6–7 Å thickness) as a consequence of strong CH–p interactions. Undecenyl resorc[4]arene 1a, which featured the simplest pattern of substituents, was submitted to olefin metathesis using the second generation Grubbs complex as the catalyst. Depending on the reaction conditions, different products were isolated: a bicyclic alkene 2a (46%), a linear dimer 3a (5%), and a cross-linked homopolymer P1a (44%).
ir conformation of 1a was confirmed by X-ray diffraction analysis, which also showed a peculiar self-assembly behavior in the crystal lattice, forming intercalated hydrophilic and hydrophobic layers (6–7 Å thickness) as a consequence of strong CH–p interactions. Undecenyl resorc[4]arene 1a, which featured the simplest pattern of substituents, was submitted to olefin metathesis using the second generation Grubbs complex as the catalyst. Depending on the reaction conditions, different products were isolated: a bicyclic alkene 2a (46%), a linear dimer 3a (5%), and a cross-linked homopolymer P1a (44%).
(RSC Adv., 2013, 3, 17567–17576).