WEB & MOBILE
Lorem ipsum dolor sit amet, dolor adipisicing dolor sit amet elit atis unde o
Plants represent a rich source of structurally diverse secondary metabolites, which can be exploited in the development of new clinically important compounds. 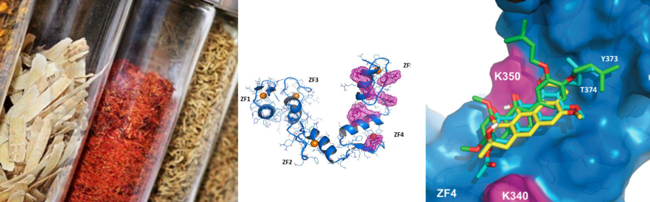 Indeed, due to their biodiversity, medicinal plants stand for the largest library of compounds that has ever existed. To date less than 1% of this vast biodiversity has been investigated in the drug discovery field, due to several factors, including the lack of a proper multidisciplinary view. Today, the collection consists of natural products belonging to different classes (variably substituted flavonoids, benzophenones, xanthones, anthraquinones, alkaloids, steroids, terpenoids, etc.) of organic compounds fully characterized. It exhibits a wide range of pharmacophores, a high degree of stereochemistry and a large range of molecular weights. These properties strongly contribute to the ability of the collection to provide bioactive compounds. It was then enlarged by the addition of other natural small molecules from commercially available sources and of synthetic or semi‐synthetic derivatives. One of the main peculiarities of this collection is that around 30% of the library compounds are classified as unique, i.e. they are not available from other commercial or literature sources. Currently, all components of this collection are incorporated into an informatic version of the library, and their chemical and physicochemical features are analysed by means of cheminformatics tools, showing a satisfactory chemical diversity. During the last ten years, the in house library offered a unique chance to identify unexpected new scaffolds for the development of therapeutically relevant molecules, providing some lead compounds that have been developed and, in some cases, patented as anticancer and antimicrobial agents. Furthermore, it is still successfully screened in silico and in vitro for the identification of hit and lead compounds in previous early-stage drug discovery projects. Efficient and versatile strategy were developed for the preparation of the synthetic and semisynthetic derivatives obtained by the optimization process of active Hits up to Lead compounds or, Lead candidates by improving potency, stability, physicochemical features (i.e. water solubility, logP, polar surface area PSA, etc…), chemical properties, and metabolic/pharmacokinetics parameters.
Indeed, due to their biodiversity, medicinal plants stand for the largest library of compounds that has ever existed. To date less than 1% of this vast biodiversity has been investigated in the drug discovery field, due to several factors, including the lack of a proper multidisciplinary view. Today, the collection consists of natural products belonging to different classes (variably substituted flavonoids, benzophenones, xanthones, anthraquinones, alkaloids, steroids, terpenoids, etc.) of organic compounds fully characterized. It exhibits a wide range of pharmacophores, a high degree of stereochemistry and a large range of molecular weights. These properties strongly contribute to the ability of the collection to provide bioactive compounds. It was then enlarged by the addition of other natural small molecules from commercially available sources and of synthetic or semi‐synthetic derivatives. One of the main peculiarities of this collection is that around 30% of the library compounds are classified as unique, i.e. they are not available from other commercial or literature sources. Currently, all components of this collection are incorporated into an informatic version of the library, and their chemical and physicochemical features are analysed by means of cheminformatics tools, showing a satisfactory chemical diversity. During the last ten years, the in house library offered a unique chance to identify unexpected new scaffolds for the development of therapeutically relevant molecules, providing some lead compounds that have been developed and, in some cases, patented as anticancer and antimicrobial agents. Furthermore, it is still successfully screened in silico and in vitro for the identification of hit and lead compounds in previous early-stage drug discovery projects. Efficient and versatile strategy were developed for the preparation of the synthetic and semisynthetic derivatives obtained by the optimization process of active Hits up to Lead compounds or, Lead candidates by improving potency, stability, physicochemical features (i.e. water solubility, logP, polar surface area PSA, etc…), chemical properties, and metabolic/pharmacokinetics parameters.
Hedgehog (Hh) signalling is a morphogenetic pathway that has a crucial role during embryonic development and tissues homeostasis. Further, Hh signaling is dysregulated in diverse types of cancer. Aberrant activation of Hedgehog signalling is the result of genetic mutations of pathway components, as the main upstream transmembrane receptor Smoothened (Smo), or other Smo-dependent or independent mechanisms, all triggering the downstream effector Gli1, which promote a gene expression pattern relevant to tumorigenesis (proliferation, survival and angiogenesis).
To identify natural products chemotypes of Smo antagonists, structure-based methods, such as docking and MD simulations, are used to screen in an in house library of natural and synthetic compounds silico against the crystallographic structure of Smo bound to Cyclopamine. We recognized seventeen potential Smo antagonists, which show a noticeable range of chemical diversity and sources. Particularly, the 2’,4’,5’,3,4- pentamethoxychalcone, was the most potent Hh inhibitor identified herein, and proved to impair the growth of Hh dependent tumor cells in vitro and in vivo.
To understand whether Gli1 binding to DNA and function could be regulated by small molecules, we performed molecular docking of an in house library toward the MD representative Gli1ZF structure. We identified six molecules putatively behaving as potential Gli1 inhibitors, among them we focused our studies on GlaB. Further we investigated the structural requirements of GLI1/DNA interaction and the role of GlaB ring-B. These studies allowed the preparation of a new generation derivatives of GlaB, which would be more selective and potents as Gli1 inhibitors.
Synergistic inhibition of the Hedgehog pathway by newly designed Smo and Gli antagonists bearing the isoflavone scaffold: Aberrant activation of the Hedgehog (Hh) pathway is responsible for the onset and progression of several malignancies. Small molecules able to block 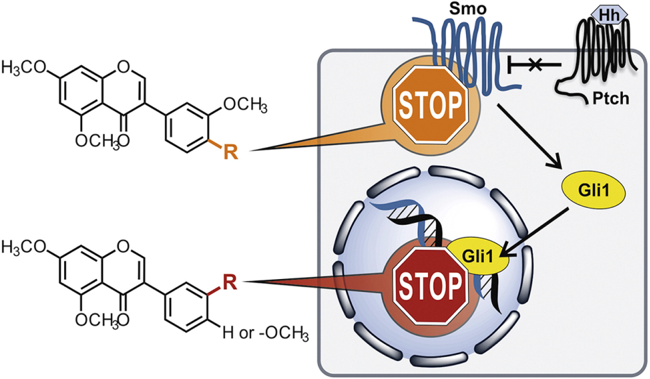 the pathway at the upstream receptor Smoothened (Smo) or the downstream effector Gli1 have thus emerged recently as valuable anticancer agents. Here, we have designed, synthesized, and tested new Hh inhibitors taking advantage by the highly versatile and privileged isoflavone scaffold. The introduction of specific substitutions on the isoflavone's ring B allowed the identification of molecules targeting preferentially Smo or Gli1. Biological assays coupled with molecular modeling corroborated the design strategy, and provided new insights into the mechanism of action of these molecules. The combined administration of two different isoflavones behaving as Smo and Gli antagonists, respectively, in primary medulloblastoma (MB) cells highlighted the synergistic effects of these agents, thus paving the way to further and innovative strategies for the pharmacological inhibition of Hh signaling.
the pathway at the upstream receptor Smoothened (Smo) or the downstream effector Gli1 have thus emerged recently as valuable anticancer agents. Here, we have designed, synthesized, and tested new Hh inhibitors taking advantage by the highly versatile and privileged isoflavone scaffold. The introduction of specific substitutions on the isoflavone's ring B allowed the identification of molecules targeting preferentially Smo or Gli1. Biological assays coupled with molecular modeling corroborated the design strategy, and provided new insights into the mechanism of action of these molecules. The combined administration of two different isoflavones behaving as Smo and Gli antagonists, respectively, in primary medulloblastoma (MB) cells highlighted the synergistic effects of these agents, thus paving the way to further and innovative strategies for the pharmacological inhibition of Hh signaling.
(European Journal of Medicinal Chemistry, 2018, 156, 554-562).
Chemical, computational and functional insights into the chemical stability of the Hedgehog pathway inhibitor GANT61: This work aims at elucidating the mechanism and kinetics of hydrolysis of GANT61, the first and mostwidely used inhibitor of the Hedgehog (Hh) 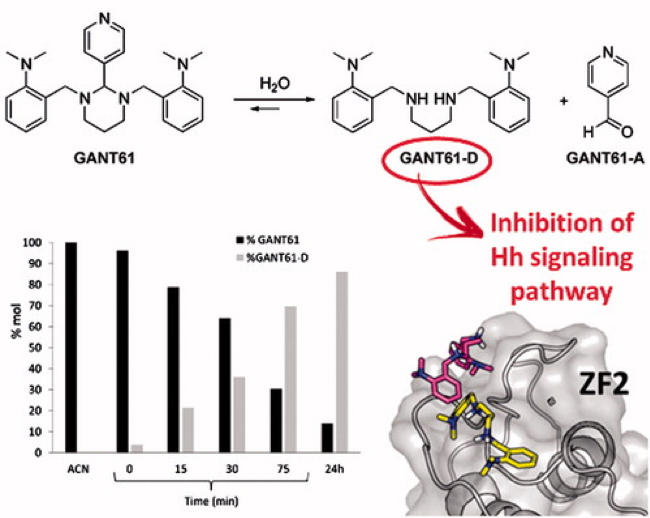 signalling pathway that targets Glioma-associated oncogene homologue (Gli) proteins, and at confirming the chemical nature of its bioactive form. GANT61 is poorly stable under physiological conditions and rapidly hydrolyses into an aldehyde species (GANT61-A), which is devoid of the biological activity against Hh signalling, and a diamine derivative (GANT61-D), which has shown inhibition of Gli-mediated transcription. Here, we combined chemical synthesis, NMR spectroscopy, analytical studies, molecular modelling and functional cell assays to characterise the GANT61 hydrolysis pathway. Our results show that GANT61-D is the bioactive form of GANT61 in NIH3T3 Shh-Light II cells and SuFu_-/_- mouse embryonic fibroblasts, and clarify the structural requirements for GANT61-D binding to Gli1. This study paves the way to the design of GANT61 derivatives with improbe potency and chemical stability
signalling pathway that targets Glioma-associated oncogene homologue (Gli) proteins, and at confirming the chemical nature of its bioactive form. GANT61 is poorly stable under physiological conditions and rapidly hydrolyses into an aldehyde species (GANT61-A), which is devoid of the biological activity against Hh signalling, and a diamine derivative (GANT61-D), which has shown inhibition of Gli-mediated transcription. Here, we combined chemical synthesis, NMR spectroscopy, analytical studies, molecular modelling and functional cell assays to characterise the GANT61 hydrolysis pathway. Our results show that GANT61-D is the bioactive form of GANT61 in NIH3T3 Shh-Light II cells and SuFu_-/_- mouse embryonic fibroblasts, and clarify the structural requirements for GANT61-D binding to Gli1. This study paves the way to the design of GANT61 derivatives with improbe potency and chemical stability
(Journal of Enzyme Inhibition and Medicinal Chemistry, (2018), 33 (1), pp. 349-358).
Polymeric glabrescione B nanocapsules for passive targeting of Hedgehog-dependent tumor therapy in vitro: With the purpose of delivering high doses of glabrescione B (GlaB) to solid tumors after systemic administration, long-circulating GlaB-loaded oil-cored polymeric 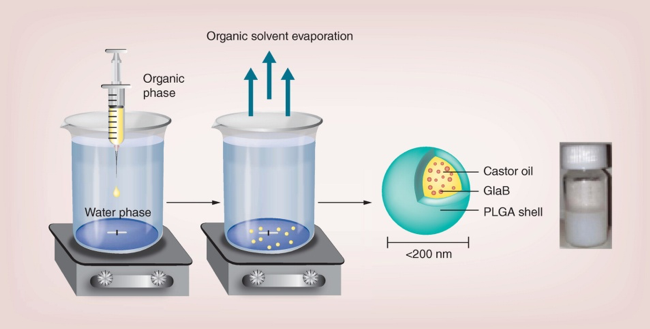 nanocapsules (NC-GlaB) were formulated. Materials & methods: Synthesis of GlaB and its encapsulation in nanocapsules (NCs) was performed. Empty- and GlaB-loaded NCs were assessed for their physico-chemical properties, in vitro cytotoxicity and in vivo biodistribution. Results: GlaB was efficiently loaded into NCs (~90%), which were small (~160 nm), homogeneous and stable upon storage. Further, GlaB and NC-GlaB demonstrated specific activities against the cancer stem cells. Preliminary studies in tumor-bearing mice supported the ability of NC to accumulate in pancreatic tumors. Conclusion: This study provides early evidence that NC-GlaB has the potential to be utilized in a preclinical setting and justifies the need to perform therapeutic experiments in mice
nanocapsules (NC-GlaB) were formulated. Materials & methods: Synthesis of GlaB and its encapsulation in nanocapsules (NCs) was performed. Empty- and GlaB-loaded NCs were assessed for their physico-chemical properties, in vitro cytotoxicity and in vivo biodistribution. Results: GlaB was efficiently loaded into NCs (~90%), which were small (~160 nm), homogeneous and stable upon storage. Further, GlaB and NC-GlaB demonstrated specific activities against the cancer stem cells. Preliminary studies in tumor-bearing mice supported the ability of NC to accumulate in pancreatic tumors. Conclusion: This study provides early evidence that NC-GlaB has the potential to be utilized in a preclinical setting and justifies the need to perform therapeutic experiments in mice
(Nanomedicine (Lond.), 2017, 12, 711–728).
Inhibition of Hedgehog-dependent tumors and cancer stem cells by a newly identified naturally occurring chemotype: Hedgehog (Hh) inhibitors have emerged as valid tools in the treatment of a wide range of cancers. Indeed, aberrant activation of the Hh pathway occurring 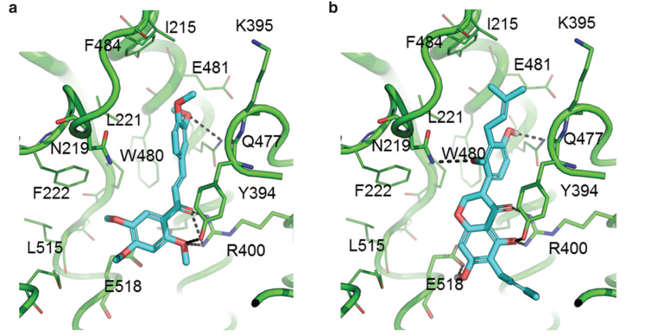 either by ligand-dependent or -independent mechanisms is a key driver in tumorigenesis. The smoothened (Smo) receptor is one of the main upstream transducers of the Hh signaling and is a validated target for the development of anticancer compounds, as underlined by the FDA-approved Smo antagonist Vismodegib (GDC-0449/Erivedge) for the treatment of basal cell carcinoma. However, Smo mutations that confer constitutive activity and drug resistance have emerged during treatment with Vismodegib. For this reason, the development of new effective Hh inhibitors represents a major challenge for cancer therapy. Natural products have always represented a unique source of lead structures in drug discovery, and in recent years have been used to modulate the Hh pathway at multiple levels. Here, starting from an in house library of natural compounds and their derivatives, we discovered novel chemotypes of Hh inhibitors by mean of virtual screening against the crystallographic structure of Smo. Hh functional based assay identified the chalcone derivative 12 as the most effective Hh inhibitor within the test set. The chalcone 12 binds the Smo receptor and promotes the displacement of Bodipy-Cyclopamine in both Smo WT and drug-resistant Smo mutant. Our molecule stands as a promising Smo antagonist able to specifically impair the growth of Hh-dependent tumor
either by ligand-dependent or -independent mechanisms is a key driver in tumorigenesis. The smoothened (Smo) receptor is one of the main upstream transducers of the Hh signaling and is a validated target for the development of anticancer compounds, as underlined by the FDA-approved Smo antagonist Vismodegib (GDC-0449/Erivedge) for the treatment of basal cell carcinoma. However, Smo mutations that confer constitutive activity and drug resistance have emerged during treatment with Vismodegib. For this reason, the development of new effective Hh inhibitors represents a major challenge for cancer therapy. Natural products have always represented a unique source of lead structures in drug discovery, and in recent years have been used to modulate the Hh pathway at multiple levels. Here, starting from an in house library of natural compounds and their derivatives, we discovered novel chemotypes of Hh inhibitors by mean of virtual screening against the crystallographic structure of Smo. Hh functional based assay identified the chalcone derivative 12 as the most effective Hh inhibitor within the test set. The chalcone 12 binds the Smo receptor and promotes the displacement of Bodipy-Cyclopamine in both Smo WT and drug-resistant Smo mutant. Our molecule stands as a promising Smo antagonist able to specifically impair the growth of Hh-dependent tumor
(Cell Death and Disease, 2016, 7, e2376).
Targeting Gli factors to inhibit the Hedgehog pathway: Hedgehog signaling is essential for tissue development and stemness, and its deregulation has been observed in many tumors. Aberrant activation of Hedgehog signaling is the result of genetic mutations of pathway 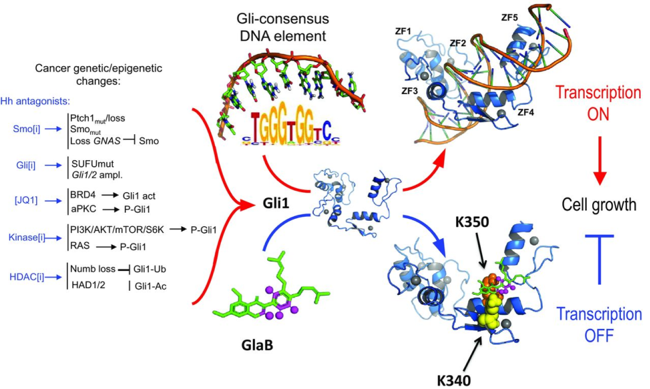 components or other Smo‐dependent or independent mechanisms, all triggering the downstream effector Gli1. For this reason, understanding the poorly elucidated mechanism of Gli1‐mediated transcription allows to identify novel molecules blocking the pathway at a downstream level, representing a critical goal in tumor biology. Here, we clarify the structural requirements of the pathway effector Gli1 for binding to DNA and identify Glabrescione B as the first small molecule binding to Gli1 zinc finger and impairing Gli1 activity by interfering with its interaction with DNA. Remarkably, as a consequence of its robust inhibitory effect on Gli1 activity, Glabrescione B inhibited the growth of Hedgehog‐dependent tumor cells in vitro and in vivo as well as the self‐renewal ability and clonogenicity of tumor‐derived stem cells. The identification of the structural requirements of Gli1/DNA interaction highlights their relevance for pharmacologic interference of Gli signaling
components or other Smo‐dependent or independent mechanisms, all triggering the downstream effector Gli1. For this reason, understanding the poorly elucidated mechanism of Gli1‐mediated transcription allows to identify novel molecules blocking the pathway at a downstream level, representing a critical goal in tumor biology. Here, we clarify the structural requirements of the pathway effector Gli1 for binding to DNA and identify Glabrescione B as the first small molecule binding to Gli1 zinc finger and impairing Gli1 activity by interfering with its interaction with DNA. Remarkably, as a consequence of its robust inhibitory effect on Gli1 activity, Glabrescione B inhibited the growth of Hedgehog‐dependent tumor cells in vitro and in vivo as well as the self‐renewal ability and clonogenicity of tumor‐derived stem cells. The identification of the structural requirements of Gli1/DNA interaction highlights their relevance for pharmacologic interference of Gli signaling
(The EMBO Journal, 2015, 34, 200-217).
The Notch signaling pathway is an inter-cellular communication system driving many biological processes in a wide spectrum of organisms. Deregulated Notch signaling due to either gene mutation or amplification, or to post-translational modifications, contributes to development and progression of different solid and hematological cancers, including T-cell acute lymphoblastic leukemia (T-ALL)3, by directly driving the expression of several oncogenes and cell cycle-related factors, and indirectly by cross-talking with other critical oncogenic signaling pathways.
A diversity-oriented random selection (DORS) of an in house library of natural products was performed by means of a clustering algorithm, which is based on a combination of fingerprints and common substructure. Among the representative molecules of the eight most abundant clusters, by functional and biological analysis we individuated the chalcone scaffold as putative Notch inhibitor molecule. In particular, we have designed and synthetized the chalcone-derivative 8 possessing Notch inhibitory activity at low micro molar concentration in T-ALL cell lines.
Identification of a novel chalcone derivative that inhibits Notch signaling in T-cell acute lymphoblastic leukemia: Notch signaling is considered a rational target in the therapy of several cancers, particularly those harbouring Notch gain of function mutations, including T-cell 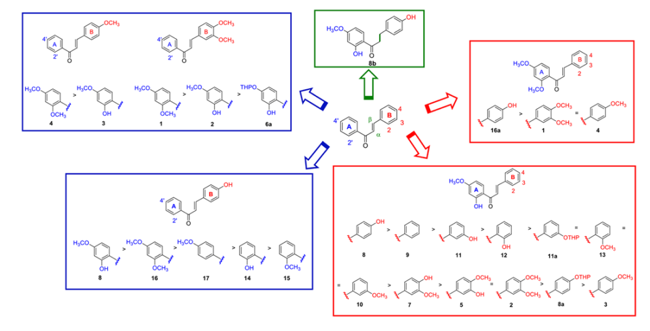 acute lymphoblastic leukemia (T-ALL). Although currently available Notch-blocking agents are showing anti-tumor activity in preclinical studies, they are not effective in all the patients and often cause severe side-effects, limiting their widespread therapeutic use. Here, by functional and biological analysis of the most representative molecules of an in house library of natural products, we have designed and synthetized the chalcone-derivative 8 possessing Notch inhibitory activity at low micro molar concentration in T-ALL cell lines. Structure-activity relationships were afforded for the chalcone scaffold. Short term treatments with compound 8 resulted in a dose-dependent decrease of Notch signaling activity, halted cell cycle progression and induced apoptosis, thus affecting leukemia cell growth. Taken together, our data indicate that 8 is a novel Notch inhibitor, candidate for further investigation and development as an additional therapeutic option against Notch-dependent cancers.
acute lymphoblastic leukemia (T-ALL). Although currently available Notch-blocking agents are showing anti-tumor activity in preclinical studies, they are not effective in all the patients and often cause severe side-effects, limiting their widespread therapeutic use. Here, by functional and biological analysis of the most representative molecules of an in house library of natural products, we have designed and synthetized the chalcone-derivative 8 possessing Notch inhibitory activity at low micro molar concentration in T-ALL cell lines. Structure-activity relationships were afforded for the chalcone scaffold. Short term treatments with compound 8 resulted in a dose-dependent decrease of Notch signaling activity, halted cell cycle progression and induced apoptosis, thus affecting leukemia cell growth. Taken together, our data indicate that 8 is a novel Notch inhibitor, candidate for further investigation and development as an additional therapeutic option against Notch-dependent cancers.
(Scientific Reports, 2017, 7, 1-13).
Colistin is a last-line antibiotic for the treatment of multidrug resistant Gram-negative bacterial infections.1 Recently, a natural ent-beyerene diterpene was identified as a promising inhibitor of the enzyme responsible for colistin resistance mediated by lipid A aminoarabinosylation in Gram-negative bacteria, namely, ArnT (undecaprenyl phosphate-alpha-4-amino-4-deoxy-l-arabinose arabinosyl transferase).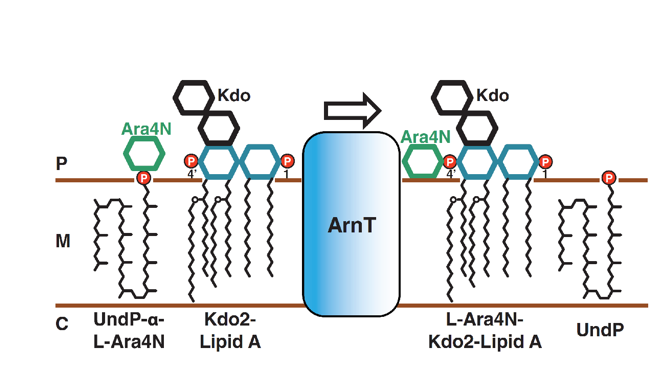 To explore structure–activity relationship (SAR) and validate the versatility of the diterpene scaffold as a key platform for further development of ArnT-mediated colistin resistance inhibitors with improved activity, a library of semisynthetic analogues of hit were designed, synthesized and tested against colistin-resistant Pseudomonas aeruginosa strains, including clinical isolates. Microbiological assays coupled with molecular modeling demonstrated that an ent-beyerane scaffold bearing an oxalate like group at C-18/C-19, or a sugar residue at C-19 to resemble L-Ara4N is an essential requirement for a more efficient inhibition of bacterial growth likely resulting from a more efficient inhibition of ArnT activity. Importantly, the easy accessibility of ent-beyerane scaffold from Stevia rebaudiana secondary metabolites will provide a cost-effective key platform for the development of promising colistin resistance inhibitors. (Journal of Organic Chemistry 2020, 85 (16), pp. 10891-10901; Journal of Antimicrobial Chemotherapy 2020, 75 (9), pp. 2564-2572).
To explore structure–activity relationship (SAR) and validate the versatility of the diterpene scaffold as a key platform for further development of ArnT-mediated colistin resistance inhibitors with improved activity, a library of semisynthetic analogues of hit were designed, synthesized and tested against colistin-resistant Pseudomonas aeruginosa strains, including clinical isolates. Microbiological assays coupled with molecular modeling demonstrated that an ent-beyerane scaffold bearing an oxalate like group at C-18/C-19, or a sugar residue at C-19 to resemble L-Ara4N is an essential requirement for a more efficient inhibition of bacterial growth likely resulting from a more efficient inhibition of ArnT activity. Importantly, the easy accessibility of ent-beyerane scaffold from Stevia rebaudiana secondary metabolites will provide a cost-effective key platform for the development of promising colistin resistance inhibitors. (Journal of Organic Chemistry 2020, 85 (16), pp. 10891-10901; Journal of Antimicrobial Chemotherapy 2020, 75 (9), pp. 2564-2572).